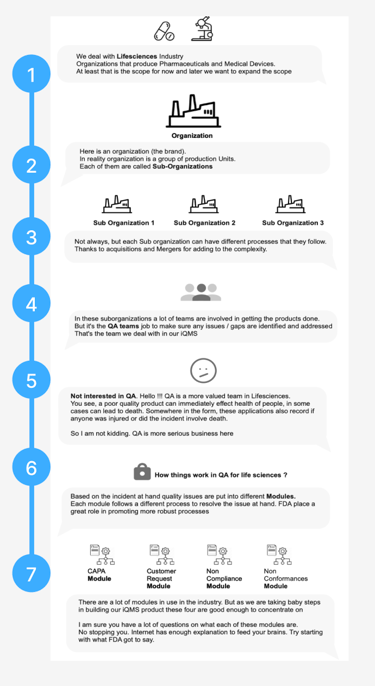
Quality Management System for LifeSciences
Project Brief
Create the most efficient path to quality excellence by delivering the exact expertise, process, and technology required by life science companies.
Help life sciences companies create healthier, safer products by easing the path to regulatory compliance.
About
Quick Summery
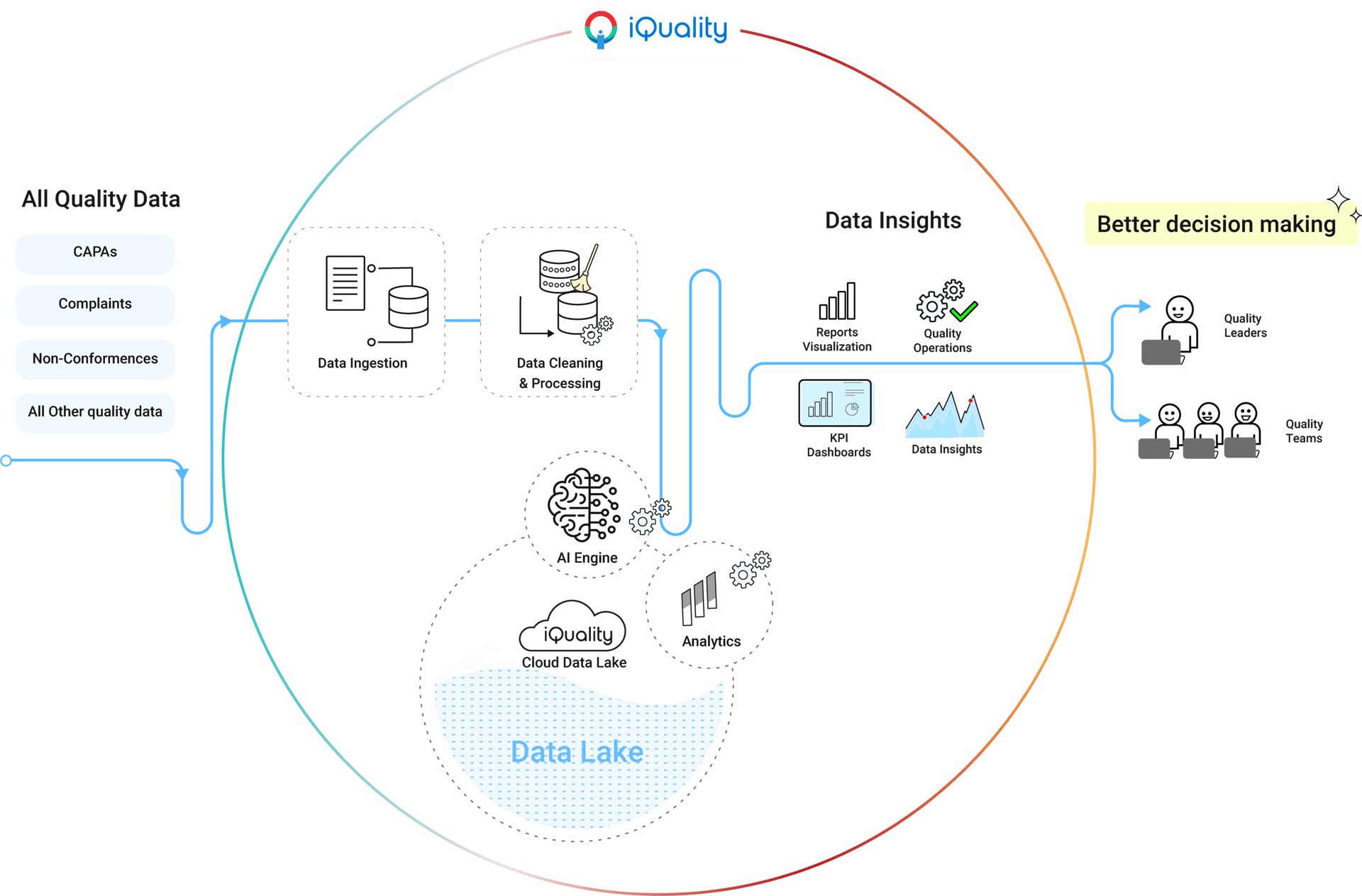
Core Functionalities
Deviation Tracking: Identifies and documents process deviations, linked to corrective actions (CAR)
Audit & Compliance: Includes tools for internal audits, documentation control, and validation protocols critical for FDA/EU MDR compliance
Risk Management: Embedded tools for risk assessment and CAPA management to address non-conformities proactively
Industry-Specific Features
Success Metrics
40%
45%
60%
Overall Efficiency
Increase
Reduction in test planning time
Reduction in summery reporting time
Design Process


User Interviews
Understand industry specific pain points, priorities, reservations.
End users - QA Teams, Manufacturing Staff in LifeSciences industries
FDA audit process experts
Initially most of the team members in the startup had no idea of the users we are building our quality management for.To the end of this exercise we were able to come-up with a user story, personas, domain specific task flows that all of us understood.
Design Contributions
As UX Design Lead at the startup, I spearheaded the end-to-end user experience strategy and execution. Collaborated closely with stakeholders to brainstorm requirements, structure the information architecture (IA), and identify effective design solutions. Through competitor analysis and user research, I curated detailed user stories that informed our design decisions and helped build a sustainable design framework.Contributed to shaping the brand identity and establishing a comprehensive design system to ensure consistency and scalability.
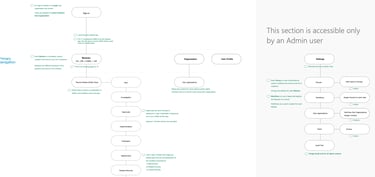
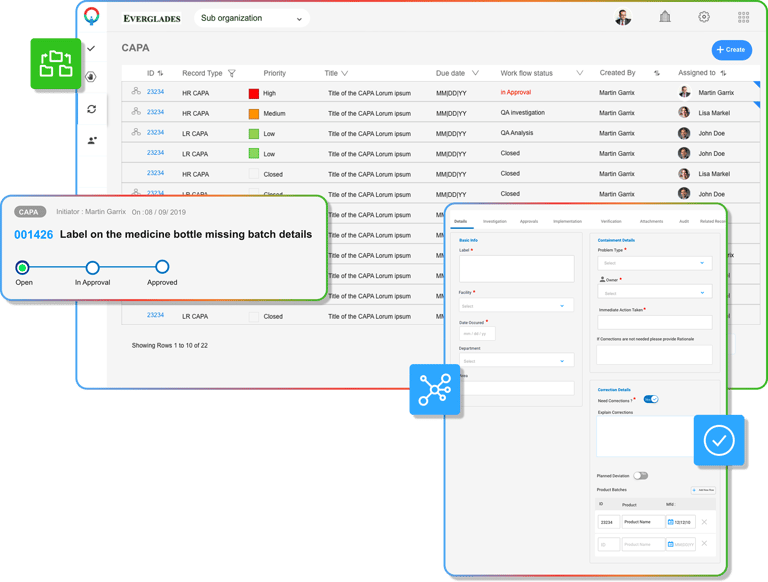
List of modules Contributed to
Here is a list of some modules that I have worked on for Compliance group - iQuality QMS tool
QMS specific Modules for Life-sciences
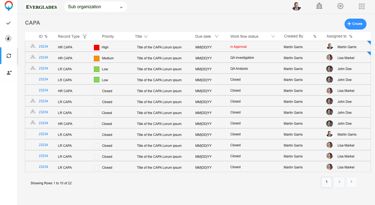
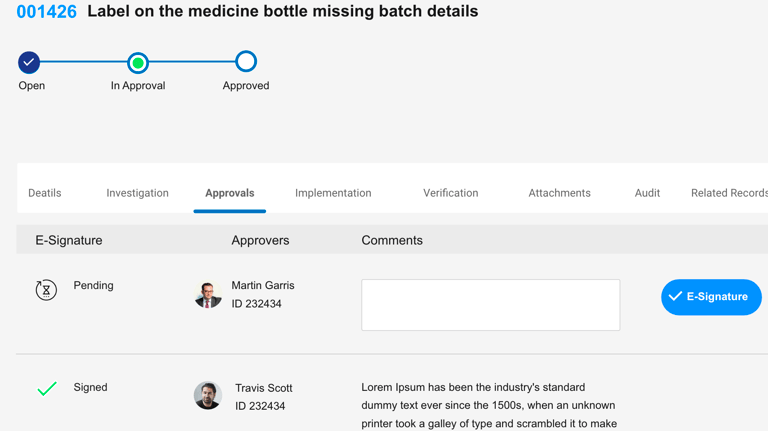
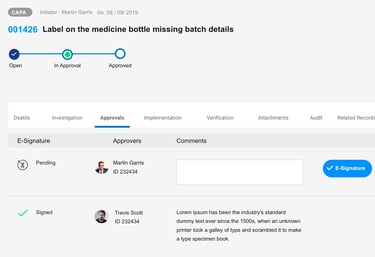
CAPA
CA
Preventive Action
Complaints
CAPA & MAUD audit reports
Operations
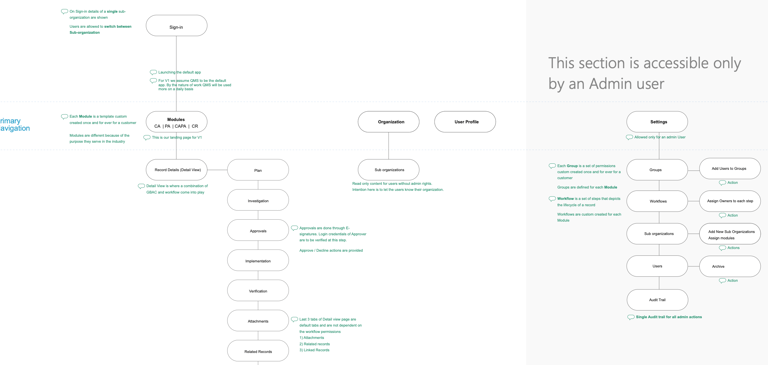
Login, Signup, Forgot Password Flows
Dashboard outlining active tickets
AI generated Reports
User & Access Management
In-App Notifications
Branding Configuration (White Labelled Portal, Emails, Invoices, Checkout)
User Profile
Settings
Design Specifications
Empty States
Error States
Interactions Specs
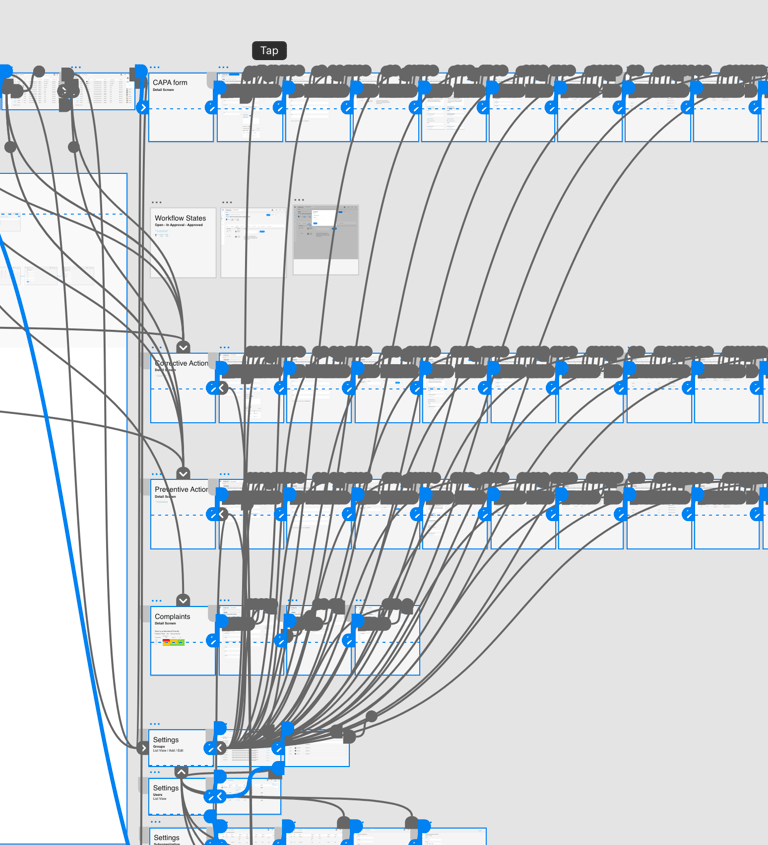
Design System
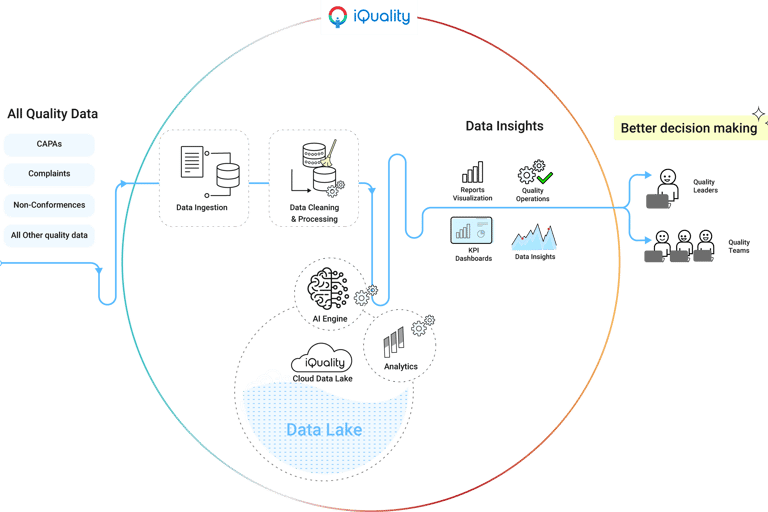
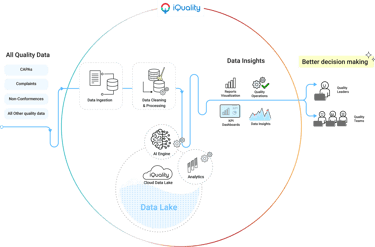
Design deliverables
Delivered UX assets including wireframes, high-fidelity designs, and interactive prototypes, design system. Worked closely with development teams to foster shared understanding of the product vision, ensuring seamless implementation and alignment between design and technical execution.
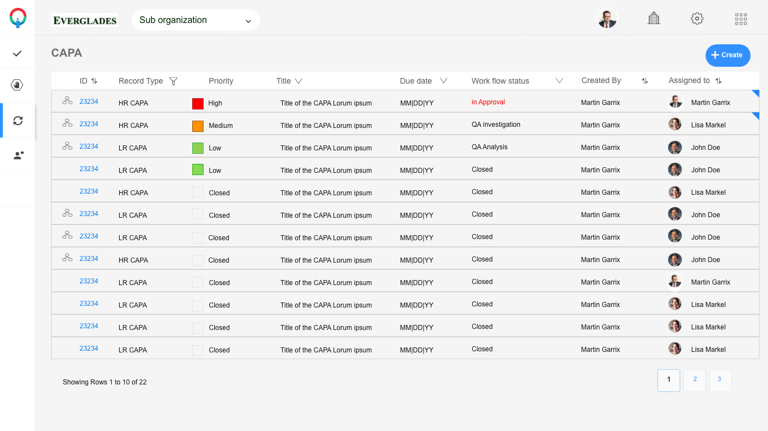

Screen Prototypes
Screen Interactions
UX Story of iQMS
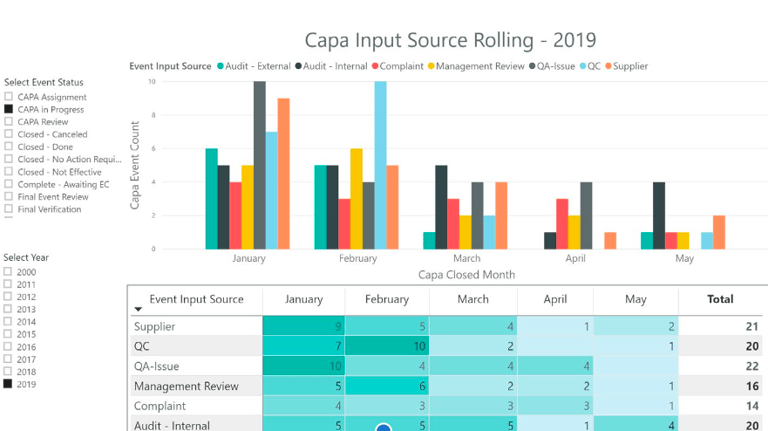
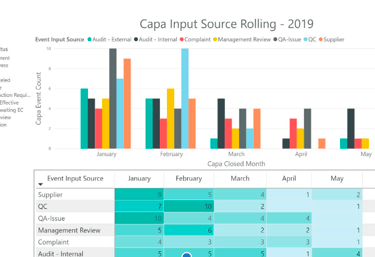
Success Metrics
UX bridges the gap between regulatory requirements and user behavior. By focusing on intuitive design and measurable efficiency gains
40%
45%
Reduction in test planning time
Overall Efficiency
Increase
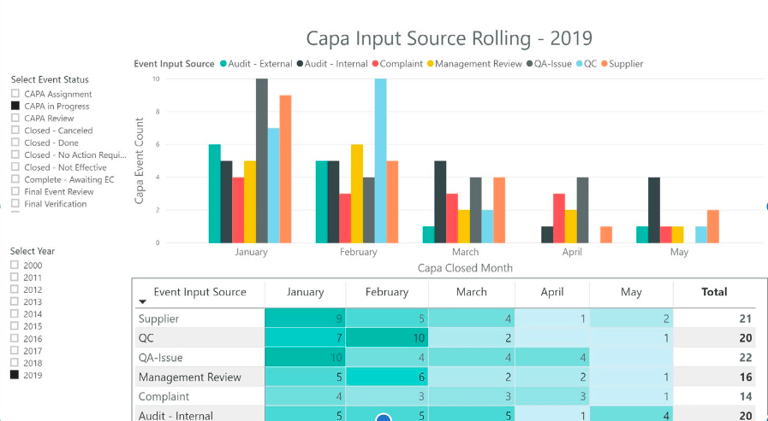
60%
Reduction in summery reporting time
AI assisted predictions of compliance event for upcoming quarters
Winning trust of key industry expert collaborators
Doubts on our AI-powered QMS approach, questioning its alignment with industry standards and practical quality management needs. Their skepticism risked delaying collaboration and credibility.
Action Plan: Bridging the Gap
Stakeholder Interviews: Conducted repeated 1:1 sessions to understand their pain points, priorities, and reservations.
User-Centric Research: Analyzed workflows of target users (e.g., QA teams, auditors) to identify gaps our AI could solve.
Market Validation: Benchmarking against competitors and showcasing case studies to prove our solution’s uniqueness.
Iterative Feedback: Shared prototypes early, incorporated expert input into product design, and demonstrated responsiveness.
Result: Trust Through Evidence
Collaborator Endorsement: Earned praise for “Hitting the ball out of the park” by addressing their core concerns with early design proof of conscepts and information visualization insights.
Strategic Alignment: Transformed skepticism into advocacy, securing their partnership for pilot programs and industry conferences.
Product Refinement: Integrated expert feedback to enhance features like audit automation and Robust risk analytics.
Key Takeaway
Trust is built through active listening, transparency, and proof of competence.
